
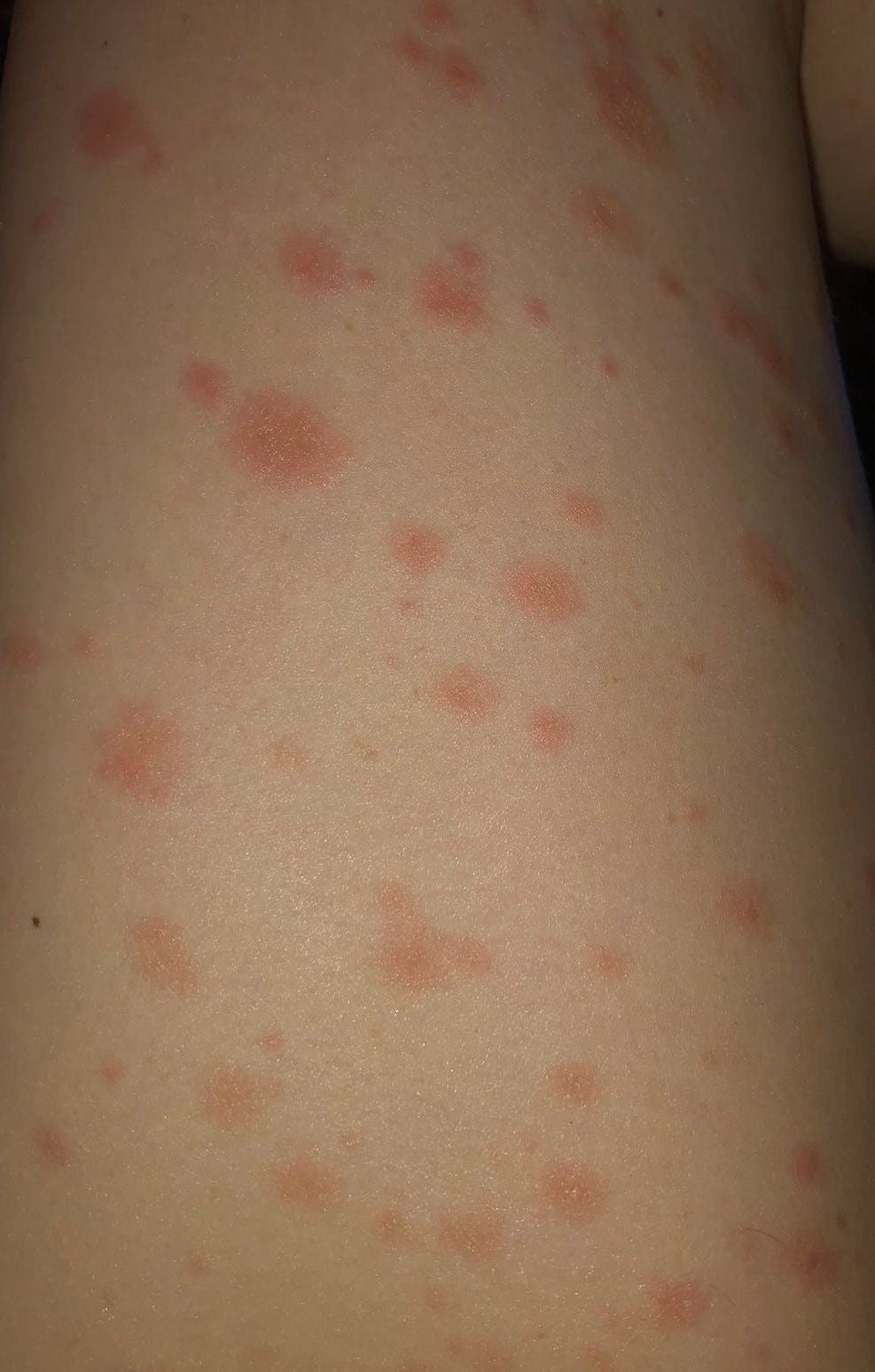
Beginning in June 2021, cases of myocarditis and myopericarditis (hereafter, myocarditis) after receipt of Pfizer-BioNTech vaccine began to be reported, primarily among young males after receipt of the second dose ( 4, 5).
#Covid vaccine side effect statistics us trial#
The Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for Pfizer-BioNTech vaccine for use in persons aged ≥16 years on Decem( 1) the EUA was expanded to include adolescents aged 12–15 years on ( 2), based on results from a Phase 3 clinical trial ( 3). Until studies have adequately evaluated the safety of CoronaVac in pregnant individuals, the WHO recommends its use when the benefits of vaccination outweigh the potential risks.Īs with other authorized COVID-19 vaccines, individuals with a history of anaphylaxis to any of the vaccine’s ingredients should not receive the vaccine.As of July 30, 2021, among the three COVID-19 vaccines authorized for use in the United States, only the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine is authorized for adolescents aged 12–17 years. The WHO also notes that while this vaccine has not been sufficiently tested in pregnant people, its similarity to other vaccines deemed safe, such as those for hepatitis B, suggests that there are no expected concerns for the use of the vaccine during pregnancy.

It states that while the trials reviewed by SAGE did not include this specific population, CoronaVac is a nonreplicating vaccine and can be recommended. The WHO also recommends CoronaVac for people living with HIV. Similar to other COVID-19 vaccines approved by WHO, the global health agency recommends the CoronaVac vaccine for people with comorbidities, those who are immunocompromised, or those who previously have had COVID-19. It uses a dead version of the SARS-CoV-2 virus so that it cannot replicate, but it keeps the surface spike protein intact to trigger the body’s immune system to create antibodies for protection against the live virus, if it were to invade. The Strategic Advisory Group of Experts on Immunization (SAGE), WHO’s main advisory board regarding vaccines, also reviewed the vaccine as part of WHO’s EUL validation.Īs of January 2022, the vaccine is approved for use in 52 countries.ĬoronaVac is an inactivated vaccine. WHO’s EUL procedure for CoronaVac included a review of the vaccine’s safety and efficacy, as well as “onsite inspections of the production facility.”

Sinovac’s vaccine was validated for Emergency Use Listing (EUL) by the WHO on June 1. It has an efficacy rate of 50.4% for preventing symptomatic infection, according to data from a Brazilian trial, and an effectiveness of 67%, according to a real-world study in Chile. This two-dose vaccine is recommended for individuals aged 18 years and above. The company focuses specifically on the development and manufacturing of vaccines to target human infectious diseases. CoronaVac is a COVID-19 vaccine produced by Sinovac Biotech, a China-based pharmaceutical company with headquarters in Beijing.


 0 kommentar(er)
0 kommentar(er)
